

In 1987, Annette reunited with Frankie Avalon for a series of promotional concerts to promote their film Back to the Beach. She began to suffer from dizzy spells, but kept her failing health from her family.
Funicello announced in 1992 that she suffers from multiple sclerosis.[7] She had kept her condition a secret for many years, but felt it necessary to go public to combat rumors that her impaired carriage was the result of alcoholism. That same year, she was inducted as a Disney Legend.[8] In 1993, she opened the Annette Funicello Fund for Neurological Disorders at the California Community Foundation.
Funicello's best friend is Shelley Fabares. Shelley and Annette have been friends since they were young teenagers, and Shelley was a bridesmaid at Annette's first wedding.
Her autobiography, published in 1994, is A Dream Is a Wish Your Heart Makes: My Story. The title is taken from a song from the movie Cinderella. A made-for-TV movie based on the book, A Dream Is a Wish Your Heart Makes: The Annette Funicello Story, was made in 1995. In the final scene, the actress portraying Funicello, riding in a wheelchair, is turned away from the camera — turning back, it is Funicello herself, who delivers a message to a group of children. During this period she also produced her own line of teddy bears for the Annette Funicello Collectible Bear Company.[9] The last collection in the series was made in 2004. She also has her own fragrance Cello by Annette.
Funicello lost her eye-sight and was confined to a wheelchair by the mid-2000s. When she was able, she would reportedly get out with a caregiver for weekly trips to the beauty parlor and her Canoga Park neighborhood Costco store
Multiple sclerosis
From Wikipedia, the free encyclopedia
| Multiple sclerosis | |
| Classification and external resources | |
| ICD-10 | G35. |
|---|---|
| ICD-9 | 340 |
| OMIM | 126200 |
| DiseasesDB | 8412 |
| MedlinePlus | 000737 |
| eMedicine | neuro/228 oph/179 emerg/321 pmr/82 radio/461 |
| MeSH | D009103 |
Multiple sclerosis (abbreviated MS, also known as disseminated sclerosis or encephalomyelitis disseminata) is an autoimmune condition in which the immune system attacks the central nervous system, leading to demyelination.[1] Disease onset usually occurs in young adults, and it is more common in females.[2] It has a prevalence that ranges between 2 and 150 per 100,000.[3] MS was first described in 1868 by Jean-Martin Charcot.[4]
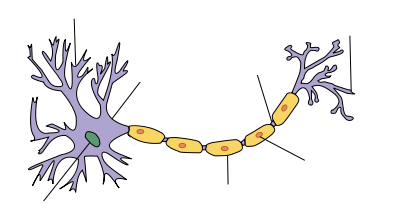
MS affects the ability of nerve cells in the brain and spinal cord to communicate with each other. Nerve cells communicate by sending electrical signals called action potentials down long fibers called axons, which are wrapped in an insulating substance called myelin. In MS, the body's own immune system attacks and damages the myelin. When myelin is lost, the axons can no longer effectively conduct signals.[1] The name multiple sclerosis refers to scars (scleroses – better known as plaques or lesions) in the white matter of the brain and spinal cord, which is mainly composed of myelin.[4] Although much is known about the mechanisms involved in the disease process, the cause remains unknown. Theories include genetics or infections. Different environmental risk factors have also been found.[1][5]
Almost any neurological symptom can appear with the disease, and often progresses to physical and cognitive disability.[1] MS takes several forms, with new symptoms occurring either in discrete attacks (relapsing forms) or slowly accumulating over time (progressive forms).[6] Between attacks, symptoms may go away completely, but permanent neurological problems often occur, especially as the disease advances.[6]
There is no known cure for MS. Treatments attempt to return function after an attack, prevent new attacks, and prevent disability.[1] MS medications can have adverse effects or be poorly tolerated, and many patients pursue alternative treatments, despite the lack of supporting scientific study. The prognosis is difficult to predict; it depends on the subtype of the disease, the individual patient's disease characteristics, the initial symptoms and the degree of disability the person experiences as time advances.[7] Life expectancy of patients is nearly the same as that of the unaffected population.[7]
Contents[hide] |
Classification
Several subtypes, or patterns of progression, have been described. Subtypes use the past course of the disease in an attempt to predict the future course. They are important not only for prognosis but also for therapeutic decisions. In 1996 the United States National Multiple Sclerosis Society standardized four subtype definitions: relapsing remitting, secondary progressive, primary progressive and progressive relapsing.[6]
The relapsing-remitting subtype is characterized by unpredictable relapses followed by periods of months to years of relative quiet (remission) with no new signs of disease activity. Deficits suffered during attacks may either resolve or leave sequelae. This describes the initial course of 85–90% of individuals with MS.[6] When deficits always resolve between attacks, this is sometimes referred to as benign MS.[8]
Secondary progressive MS describes those with initial relapsing-remitting MS, who then begin to have progressive neurologic decline between acute attacks without any definite periods of remission.[6] Occasional relapses and minor remissions may appear.[6] The median time between disease onset and conversion from relapsing-remitting to secondary progressive MS is 19 years.[9]
The primary progressive subtype describes the approximately 10–15% of individuals who never have remission after their initial MS symptoms.[10] It is characterized by progression of disability from onset, with no, or only occasional and minor, remissions and improvements.[6] The age of onset for the primary progressive subtype is later than other subtypes.[10]
Progressive relapsing MS describes those individuals who, from onset, have a steady neurologic decline but also suffer clear superimposed attacks. This is the least common of all subtypes.[6]
Cases with non-standard behavior have also been described. Sometimes referred to as borderline forms of multiple sclerosis,[11] these include Devic's disease, Balo concentric sclerosis, Schilder's diffuse sclerosis and Marburg multiple sclerosis.[12][13] Multiple sclerosis also behaves differently in children.[14] There is debate whether these are atypical variants of MS or different diseases.[15]
Signs and symptoms
Symptoms of MS usually appear in episodic acute periods of worsening (relapses, exacerbations, bouts or attacks), in a gradually progressive deterioration of neurologic function, or in a combination of both.[6]
The most common presentation of MS is the clinically isolated syndrome (CIS). In CIS, a patient has an attack suggestive of demyelination, but does not fulfill the criteria for multiple sclerosis.[16] Only 30 to 70% of persons experiencing CIS later develop MS.[16] The disease usually presents with sensorial (46% of cases), visual (33%), cerebellar (30%) and motor (26%) symptoms.[17] Many rare initial symptoms have also been reported, including aphasia, psychosis and epilepsy.[18][19][20] Patients first seeking medical attention commonly present with multiple symptoms.[17] The initial signs and symptoms of MS are often transient, mild, and self-limited. These signs and symptoms often do not prompt a person to seek medical attention and are sometimes identified only retrospectively once the diagnosis of MS has been made. Cases of MS are sometimes incidentally identified during neurological examinations performed for other causes. Such cases are referred to as subclinical MS.[21][22]
The person with MS can suffer almost any neurological symptom or sign, including changes in sensation (hypoesthesia and paraesthesia), muscle weakness, muscle spasms, or difficulty in moving;[23] difficulties with coordination and balance (ataxia);[23] problems in speech (dysarthria) or swallowing (dysphagia),[24] visual problems (nystagmus, optic neuritis, or diplopia),[25] fatigue, acute or chronic pain,[26][27] and bladder and bowel difficulties.[27][28] Cognitive impairment of varying degrees and emotional symptoms of depression or unstable mood are also common.[29][30] The main clinical measure of disability progression and symptom severity is the Expanded Disability Status Scale or EDSS.[31]
Multiple sclerosis relapses are often unpredictable, occurring without warning and without obvious inciting factors. Some attacks, however, are preceded by common triggers. Relapses occur more frequently during spring and summer.[32] Infections such as the common cold, influenza, or gastroenteritis increase the risk of relapse.[33][34] Stress may also trigger an attack.[35][36][37] Pregnancy may affect susceptibility to relapse, offering protection during the last trimester, for instance. During the first few months after delivery, however, the risk of relapse is increased. Overall, pregnancy does not seem to influence long-term disability.[38] Many potential triggers have been examined and found not to influence MS relapse rates. There is no evidence that vaccination for influenza, hepatitis B, varicella, tetanus, or tuberculosis increases risk of relapse.[39] Physical trauma does not trigger relapses.[40][41] Exposure to higher than usual ambient temperatures can exacerbate extant symptoms, an effect known as Uhthoff's phenomenon.[42] Uhthoff's phenomenon is not, however, an established relapse trigger.[32]
Causes
Epidemiological studies of MS have provided hints on possible causes for the disease. Various theories try to combine the known data into plausible explanations, but none has proved definitive. MS likely occurs as a result of some combination of both environmental and genetic factors.
Genetic factors
MS is not considered a hereditary disease. However, a number of genetic variations have been shown to increase the risk of developing the disease.[43]
The risk of acquiring MS is higher in relatives of a person with the disease than in the general population, especially in the case of siblings, parents, and children.[1] In the case of monozygotic twins, concordance occurs only in about 35% of cases, and half-siblings have a lower risk than full siblings, indicating a polygenic origin.[1][44]
Apart from familial studies, specific genes have been linked with MS. Differences in the human leukocyte antigen (HLA) system—a group of genes in chromosome 6 that serves as the major histocompatibility complex in humans—increase the probability of suffering MS.[45] Two other genes have been shown to be linked to MS. These are the IL2RA and the IL7RA, subunits of the receptor for interleukin 2 and interleukin 7 respectively.[46][47] The HLA complex is involved in antigen presentation, which is crucial to the functioning of the immune system, while mutations in the IL2 and IL7 receptor genes were already known to be associated with diabetes and other autoimmune conditions, supporting the notion that MS is an autoimmune disease.[45][48][49] The gene encoding kinesin KIF1B is the first neuronal expressed gene demonstrated to enhance the risk for the disease.[50] Other studies have linked genes in chromosome 5 with the disease.[51]
Infectious cause
Genetic susceptibility can explain some of the geographic and epidemiological variations in MS incidence, like the high appearance of the disease among some families or the risk decline with genetic distance, but does not account for other phenomena, such as the changes in risk that occur with migration at an early age.[5]
An explanation for this epidemiology finding could be that some kind of infection, produced by a widespread microbe rather than a rare pathogen, is the origin of the disease.[5] Different hypotheses have elaborated on the mechanism by which this may occur. The hygiene hypothesis proposes that exposure to several infectious agents early in life is protective against MS. MS would be an autoimmune reaction triggered in susceptible individuals by multiple infective microorganisms, with risk increasing with age at infection.[5][52][53] The prevalence hypothesis proposes that the disease is due to a pathogen more common in regions of high MS prevalence. This pathogen is very common, causing in most individuals an asymptomatic persistent infection. Only in a few cases, and after many years since the original infection, does it bring demyelination.[5][54] The hygiene hypothesis has received more support than the prevalence hypothesis.[5]
Evidence for viruses as a cause includes the presence of oligoclonal bands in the brain and cerebrospinal fluid of most patients, the association of several viruses with human demyelinating encephalomyelitis, and induction of demyelination in animals through viral infection.[55] Human herpesviruses are a candidate group of viruses linked to MS;[56] Varicella zoster virus has been found at high levels in the cerebrospinal fluid of MS patients,[57] but the most reproduced finding is the reduced risk of having the disease in those who have never been infected by the Epstein-Barr virus[5][58], together with the correlation of its markers with disease activity[59]. This goes against the hygiene hypothesis, since the non-infected have probably experienced a more hygienic upbringing.[5] Other agents that have also been related with MS are human endogenous retroviruses and Chlamydia pneumoniae.[60][61][62]
Non-infectious environmental risk factors
MS is more common in people who live farther from the equator. Decreased sunlight exposure has been linked with a higher risk of MS.[63][64][65] Decreased vitamin D production and intake has been the main biological mechanism used to explain the higher risk among those less exposed to sun.[63][66][67]
Severe stress may also be a risk factor although evidence is weak;[63] parents who lost a child unexpectedly were more likely to develop MS than parents who had not.[68] Smoking has also been shown to be an independent risk factor for developing MS.[66][69] Association with occupational exposures and toxins—mainly solvents—has been evaluated, but no clear conclusions have been reached.[63] Vaccinations were also considered as causal factors for the disease; however, most studies show no association between MS and vaccines.[63]
Gout occurs less than would statistically be expected in people with MS, and low levels of uric acid have been found in MS patients as compared to normal individuals. This led to the theory that uric acid, which can protect against oxidative stress from substances such as peroxynitrite, protects against MS, although its exact importance remains unknown.[70][71][72] Several other possible risk factors, such as diet and hormone intake, have been investigated; however, more evidence is needed to confirm or refute their relation with the disease.[66]
Although some of these risk factors, including infection, are partly modifiable, only further research—especially clinical trials—will reveal whether their elimination can help prevent MS.[73]
Pathophysiology
| Myelin sheath of a healthy neuron |
|---|
MS as an autoimmunological disease
MS is currently believed to be an immune-mediated disorder with an initial trigger, which may have a viral etiology,[1] although this concept has been debated for years and some still oppose it. Damage is believed to be caused by the patient's own immune system. The immune system attacks the nervous system, possibly as a result of exposure to a molecule with a similar structure to one of its own.[1]
Lesions
The name multiple sclerosis refers to the scars (scleroses – better known as plaques or lesions) that form in the nervous system. MS lesions most commonly involve white matter areas close to the ventricles of the cerebellum, brain stem, basal ganglia and spinal cord; and the optic nerve. The function of white matter cells is to carry signals between grey matter areas, where the processing is done, and the rest of the body. The peripheral nervous system is rarely involved.[1]
More specifically, MS destroys oligodendrocytes, the cells responsible for creating and maintaining a fatty layer—known as the myelin sheath—which helps the neurons carry electrical signals.[1] MS results in a thinning or complete loss of myelin and, as the disease advances, the cutting (transection) of the neuron's extensions or axons.[74] When the myelin is lost, a neuron can no longer effectively conduct electrical signals.[1] A repair process, called remyelination, takes place in early phases of the disease, but the oligodendrocytes cannot completely rebuild the cell's myelin sheath.[75] Repeated attacks lead to successively fewer effective remyelinations, until a scar-like plaque is built up around the damaged axons.[75] Four different lesion patterns have been described.[76]
Inflammation
Apart from demyelination, the other pathologic hallmark of the disease is inflammation. According to a strictly immunological explanation of MS, the inflammatory process is caused by T cells, a kind of lymphocyte. Lymphocytes are cells that play an important role in the body's defenses.[1] In MS, T cells gain entry into the brain via the blood–brain barrier, a capillary system that should prevent entrance of T cells into the nervous system.[1] The blood–brain barrier is normally not permeable to these types of cells, unless triggered by infection or a virus, which decreases the integrity of the tight junctions forming the barrier.[1] When the blood–brain barrier regains its integrity, usually after infection or virus has cleared, the T cells are trapped inside the brain.[1] The T cells recognize myelin as foreign and attack it as if it were an invading virus. This triggers inflammatory processes, stimulating other immune cells and soluble factors like cytokines and antibodies. Leaks form in the blood–brain barrier, which in turn cause a number of other damaging effects such as swelling, activation of macrophages, and more activation of cytokines and other destructive proteins.[1]
Diagnosis

Multiple sclerosis can be difficult to diagnose since its signs and symptoms may be similar to many other medical problems.[77] Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process for practicing physicians. Historically, the Schumacher and Poser criteria were both popular.[78] Currently, the McDonald criteria focus on a demonstration with clinical, laboratory and radiologic data of the dissemination of MS lesions in time and space. A diagnosis cannot be made until other possible conditions have been ruled out and there is evidence of demyelinating events separated anatomically and in time.[79]
Clinical data alone may be sufficient for a diagnosis of MS if an individual has suffered separate episodes of neurologic symptoms characteristic of MS.[79] Since some people seek medical attention after only one attack, other testing may hasten and ease the diagnosis. The most commonly used diagnostic tools are neuroimaging, analysis of cerebrospinal fluid and evoked potentials. Magnetic resonance imaging of the brain and spine shows areas of demyelination (lesions or plaques). Gadolinium can be administered intravenously as a contrast to highlight active plaques and, by elimination, demonstrate the existence of historical lesions not associated with symptoms at the moment of the evaluation.[79][80] Testing of cerebrospinal fluid obtained from a lumbar puncture can provide evidence of chronic inflammation of the central nervous system. The cerebrospinal fluid is tested for oligoclonal bands, which are an inflammation marker found in 75–85% of people with MS.[79][81] The nervous system of a person with MS often responds less actively to stimulation of the optic nerve and sensory nerves due to demyelination of such pathways. These brain responses can be examined using visual and sensory evoked potentials.[82]
Treatment
Although there is no known cure for multiple sclerosis, several therapies have proven helpful. The primary aims of therapy are returning function after an attack, preventing new attacks, and preventing disability. As with any medical treatment, medications used in the management of MS have several adverse effects. Alternative treatments are pursued by some patients, despite the shortage of supporting, comparable, replicated scientific study.
Management of acute attacks
During symptomatic attacks, administration of high doses of intravenous corticosteroids, such as methylprednisolone,[83][84] is the routine therapy for acute relapses. The aim of this kind of treatment is to end the attack sooner and leave fewer lasting deficits in the patient. Although generally effective in the short term for relieving symptoms, corticosteroid treatments do not appear to have a significant impact on long-term recovery.[85] Potential side effects include osteoporosis[86] and impaired memory, the latter being reversible.[87]
Disease-modifying treatments
The earliest clinical presentation of relapsing-remitting MS (RRMS) is the clinically isolated syndrome (CIS). Several studies have shown that treatment with interferons during an initial attack can decrease the chance that a patient will develop clinical MS.[88][89][90]
As of 2007, six disease-modifying treatments have been approved by regulatory agencies of different countries for RRMS. Three are interferons: two formulations of interferon beta-1a (trade names Avonex, CinnoVex, ReciGen and Rebif) and one of interferon beta-1b (U.S. trade name Betaseron, in Europe and Japan Betaferon). A fourth medication is glatiramer acetate (Copaxone). The fifth medication, mitoxantrone, is an immunosuppressant also used in cancer chemotherapy, approved only in the USA and largely for secondary progressive MS. The sixth is natalizumab (marketed as Tysabri). All six medications are modestly effective at decreasing the number of attacks and slowing progression to disability, although their efficacy rates differ, and studies of their long-term effects are still lacking.[91][92][93][94] Comparisons between immunomodulators (all but mitoxantrone) show that the most effective is natalizumab, both in terms of relapse rate reduction and halting disability progression;[95] it has also been shown to reduce the severity of MS.[96] Mitoxantrone may be the most effective of them all;[97] however, it is generally not considered as a long-term therapy, as its use is limited by severe cardiotoxicity.[98]
The interferons and glatiramer acetate are delivered by frequent injections, varying from once-per-day for glatiramer acetate to once-per-week (but intra-muscular) for Avonex. Natalizumab and mitoxantrone are given by IV infusion at monthly intervals.
Treatment of progressive MS is more difficult than relapsing-remitting MS. Mitoxantrone has shown positive effects in patients with secondary progressive and progressive relapsing courses. It is moderately effective in reducing the progression of the disease and the frequency of relapses in patients in short-term follow-up.[94] No treatment has been proven to modify the course of primary progressive MS.[99]
As with any medical treatment, these treatments have several adverse effects. One of the most common is irritation at the injection site for glatiramer acetate and the interferon treatments. Over time, a visible dent at the injection site, due to the local destruction of fat tissue, known as lipoatrophy, may develop. Interferons produce symptoms similar to influenza;[100] some patients taking glatiramer experience a post-injection reaction manifested by flushing, chest tightness, heart palpitations, breathlessness, and anxiety, which usually lasts less than thirty minutes.[92] More dangerous are liver damage from interferons and mitoxantrone,[101][102][103][104][105] the immunosuppressive effects and cardiac toxicity of the latter;[105] and the putative link between natalizumab and some cases of progressive multifocal leukoencephalopathy.[106][107][108]
Management of the effects of MS
Disease-modifying treatments reduce the progression rate of the disease, but do not stop it. As multiple sclerosis progresses, the symptomatology tends to increase. The disease is associated with a variety of symptoms and functional deficits that result in a range of progressive impairments and disability. Management of these deficits is therefore very important. Both drug therapy and neurorehabilitation have shown to ease the burden of some symptoms, though neither influences disease progression.[109] As for any patient with neurologic deficits, a multidisciplinary approach is key to limiting and overcoming disability; however, there are particular difficulties in specifying a ‘core team’ because people with MS may need help from almost any health profession or service at some point.[110] Similarly, for each symptom there are different treatment options. Treatments should therefore be individualized depending both on the patient and the physician.
Alternative treatments
As with most chronic diseases, alternative treatments are pursued by some patients, despite the shortage of supporting, comparable, replicated scientific study. Examples are dietary regimens,[111] herbal medicine, including the use of medical cannabis to help alleviate symptoms,[112][113] and hyperbaric oxygenation.[114] The therapeutic practice of martial arts such as tai chi, relaxation disciplines such as yoga, or general exercise seems to mitigate fatigue, but has no effect on cognitive function.[115]
Prognosis
The prognosis (the expected future course of the disease) for a person with multiple sclerosis depends on the subtype of the disease; the individual's sex, age, and initial symptoms; and the degree of disability the person experiences.[7] Female sex, relapsing-remitting subtype, optic neuritis or sensory symptoms at onset, few attacks in the initial years and especially early age at onset, are associated with a better course.[7][116]
The life expectancy of people with MS, at least for earlier years, is nearly the same as that of unaffected people.[7] Almost 40% of patients reach the seventh decade of life.[116] Nevertheless, half of the deaths in people with MS are directly related to the consequences of the disease, while 15% more are due to suicide, a percentage much higher than in the healthy population.[7][117]
Although most patients lose the ability to walk prior to death, 90% are still capable of independent walking at 10 years from onset, and 75% at 15 years.[116][118]
Epidemiology
Two main measures are used in epidemiological studies: incidence and prevalence. Incidence is the number of new cases per unit of person–time at risk (usually number of new cases per thousand person–years); while prevalence is the total number of cases of the disease in the population at a given time. Prevalence is known to depend not only on incidence, but also on survival rate and migrations of affected people. MS has a prevalence that ranges between 2 and 150 per 100,000 depending on the country or specific population.[3] Studies on populational and geographical patterns of epidemiological measures have been very common in MS,[54] and have led to the proposal of different etiological (causal) theories.[5][54][63][66]
MS usually appears in adults in their thirties,[2] but it can also appear in children,[119] and the primary progressive subtype is more common in people in their fifties.[10] As with many autoimmune disorders, the disease is more common in women, and the trend may be increasing.[54][120] In children, the sex ratio may reach three females for each male.[119] In people over fifty, MS affects males and females almost equally.[10]
There is a north-to-south gradient in the northern hemisphere and a south-to-north gradient in the southern hemisphere, with MS being much less common in people living near the equator.[120] Climate, sunlight and intake of vitamin D have been investigated as possible causes of the disease that could explain this latitude gradient.[66] However, there are important exceptions to the north-south pattern such as incidence and prevalence in the Canary Islands[121] and changes in prevalence rates over time;[122] in general, this trend might be disappearing.[120] This indicates that other factors such as environment or genetics have to be taken into account to explain the origin of MS.[122]
Environmental factors during childhood may play an important role in the development of MS later in life. Several studies of migrants show that if migration occurs before the age of fifteen, the migrant acquires the new region's susceptibility to MS. If migration takes place after age fifteen, the migrant retains the susceptibility of his home country.[63] However, the age–geographical risk for developing multiple sclerosis may span a larger timescale.[123]
Even in regions where MS is common, some ethnic groups are at low risk of developing the disease, including the Samis, Turkmen, Amerindians, Canadian Hutterites, Africans, and New Zealand Maoris.[63] Scotland appears to have one of the highest rates of MS in the world.[124]
History
Medical discovery

The French neurologist Jean-Martin Charcot (1825–1893) was the first person to recognize multiple sclerosis as a distinct disease in 1868. Summarizing previous reports and adding his own clinical and pathological observations, Charcot called the disease sclerose en plaques. The three signs of MS now known as Charcot's triad 1 are nystagmus, intention tremor, and telegraphic speech, though these are not unique to MS. Charcot also observed cognition changes, describing his patients as having a "marked enfeeblement of the memory" and "conceptions that formed slowly".[4]
Prior to Charcot, Robert Carswell (1793–1857), a British professor of pathology, and Jean Cruveilhier (1791–1873), a French professor of pathologic anatomy, had described and illustrated many of the disease's clinical details, but did not identify it as a separate disease.[125]
After Charcot's description, Eugène Devic (1858–1930), Jozsef Balo (1895–1979), Paul Ferdinand Schilder (1886–1940), and Otto Marburg (1874–1948) described special cases of the disease.
Historical cases
There are several historical accounts of people who lived before or shortly after the disease was described by Charcot and probably had MS.
A young woman called Halldora, who lived in Iceland around the year 1200, suddenly lost her vision and mobility, but after praying to the saints, recovered them seven days after. Saint Lidwina of Schiedam (1380–1433), a Dutch nun, may be one of the first clearly identifiable MS patients. From the age of 16 until her death at 53, she suffered intermittent pain, weakness of the legs, and vision loss—symptoms typical of MS.[126] Both cases have led to the proposal of a 'Viking gene' hypothesis for the dissemination of the disease.[127]
Augustus Frederick d'Este (1794–1848), an illegitimate grandson of King George III of Great Britain, almost certainly suffered from MS. D'Este left a detailed diary describing his 22 years living with the disease. His diary began in 1822 and ended in 1846, although it remained unknown until 1948. His symptoms began at age 28 with a sudden transient visual loss after the funeral of a friend. During the course of his disease, he developed weakness of the legs, clumsiness of the hands, numbness, dizziness, bladder disturbances, and erectile dysfunction. In 1844, he began to use a wheelchair. Despite his illness, he kept an optimistic view of life.[128][129]
Another early account of MS was kept by the British diarist W. N. P. Barbellion, nom-de-plume of Bruce Frederick Cummings (1889–1919), who maintained a detailed log of his diagnosis and struggle with MS.[129] His diary was published in 1919 as The Journal of a Disappointed Man.[130]
Research directions

A number of treatments that may curtail attacks or improve function are under investigation. Some of these treatments involve the combination of drugs that are already in use for multiple sclerosis, such as the joint administration of mitoxantrone and glatiramer acetate (Copaxone).[131] However, most treatments already in clinical trials involve drugs that are used in other diseases. These are alemtuzumab (trade name Campath),[132] daclizumab (trade name Zenapax),[133] inosine,[134] BG00012,[135] fingolimod,[136] and teriflunomide, the active metabolite of the DMARD leflunomide. Alemtuzumab performed better than interferon beta-1a in relapsing-remitting MS reducing disability, imaging abnormalities and frequence of relapses, at the cost of increased autoimmunity problems. These included three cases of thrombocytopenic purpura which led to the suspension of the therapy.[137] Other drugs in clinical trials have been designed specifically for MS, such as laquinimod,[138] and Neurovax.[139]
Low dose naltrexone has been prescribed off-label for certain autoimmune disorders, including MS, and there is anecdotal evidence of benefit,[140][141] but only two small clinical trials have been conducted (as on December 2008), one in San Francisco, USA,[142] the other for the primary progressive variety in Milan, Italy.[143]
New diagnostic and evolution evaluation methods are also being investigated. The measurement of antibodies against myelin proteins such as myelin oligodendrocyte glycoprotein and myelin basic protein could be useful for diagnosis. Optical coherence tomography of the eye's retina could be used as a measure of response to medication, axonal degeneration and brain atrophy.[144][145] Currently there are no clinically established laboratory investigations available that can predict prognosis. However, several promising approaches have been proposed, such as the measurement of a lipid-specific immunoglobulin M as predictor of long-term outcomes.[146]
I selected Annette Funicello to put a face on Multiple Sclerosis. I watched the original Mickey Mouse Club in the 1950s. Annette was one of the original Mouseketeers.
Suggested by Marina Levkovich







Nice post, but Annette doesn't live in Canoga Park.
ReplyDeleteThank you for the comment. Where does she live? Why did you put "Anonymous"? Is it possible to reveal your name?
ReplyDeleteIf you surf the Internet I'm sure you can find it. But I don't want to compromise her privacy any more than it already has been.
ReplyDeleteRita
Rita,
ReplyDeleteThank you for coming back to my post, and revealing your name. It is not my intent to know where Annette lives.
I am severely disabled. To connect a face to a disability will enhance my Caregivers' empathy when they move on to their careers.
Love, Carolyn