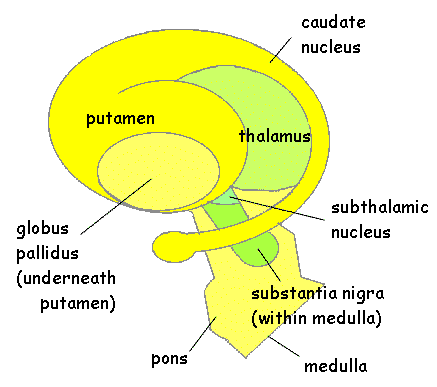
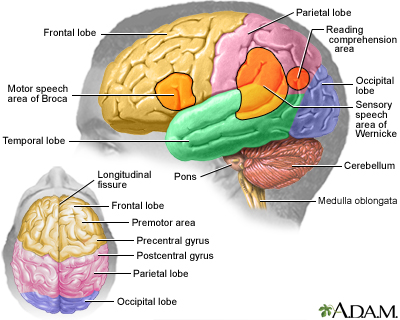
Today, Dr. Kaku addresses a question posed by Sassan K. Darian: What are the practical applications of cryogenics today, and what potential improvements can we expect 20 to 30 years down the line?
The technology itself is almost 100 years old, but it took that long to get all the bugs out and create a new product that may revolutionize the media landscape. Other companies have slowly been implementing autostereoscopic technology into their devices. In 2009, Fujifilm released the FinePix Real 3D W1 digital camera which featured a built-in autostereoscopic LCD display and a twin-CCD/twin-lens system to capture your 3D images. (I have a chapter on this and other startling new technologies in my new book, Physics of the Future, also out in March.
In the future, perhaps all video and computer screens will employ a version of this technology. This could be the next big thing! Goodbye, clunky 3D glasses. Hello, the world of the Matrix.
Basically, these games use lenticular technology, the same technology used in novelty shops to create pictures that seem to fluctuate between two images (e.g. a person with eyes open, and then eyes closed). The key to these video games is the screen. The screen consists of many vertical lines, each one shaped somewhat like a prism. These vertical lines split an image in half, with one going to the left eye, and the other going to the right eye. Hence, each eye sees a slightly different image. When the brain puts these two images together, it creates the illusion of 3D. This technology dispenses totally with the glasses, since the image is split by the screen itself, not the glasses.
This development removes the main hurdle facing 3D technology. There are some drawbacks, which should be ironed out in the coming years, as TV, computer screens, and even flexible computerized wallpaper adopt this technology. You have to be in the sweet spot in order to get this full effect, about a foot behind the screen. If you are slightly outside the sweet spot, you experience ghosting, i.e. a slightly blurred image. (This is not so much a problem with video games, but it becomes more of a problem with TV screens that have to fill up a living room.)
Another challenge is software. Even though hardware may soon exist that will allow us to view 3D movies without glasses, Hollywood has to catch up and create movies for this new medium. In addition, creating software may be a bit expensive for this new technology. In lenticular technology, two separate images are broken up into many vertical strips, and then combined digitally, so they alternate, one strip after the next. Then the lenticular lens is placed right on top of these mixed strips. The prisms in these vertical lines in the lens separate this mixture into two separate images. This requires sophisticated technology. The integration of lenticular technology is just one step in the long road to creating a Matrix.
The 3DS does come pre-loaded with its own brand of built-in software including a Mii maker, Activity Log, Internet Browser, Backwards Compatibility, Camera and even the ability to download classic Nintendo games. One interesting feature is called the StreetPass™ which the 3DS website says "is the place where Mii™ characters meet and greet! When StreetPass™ communication is activated, you can exchange Mii data, recent gameplay info, and more with other Nintendo 3DS owners you pass on the street when your Nintendo 3DS system is in Sleep Mode. You'll then be able to see these Mii characters in the plaza the next time you start playing."

The next step, mentioned in my book, is to create Internet contact lenses that can shoot two 3D images directly into the retina of each eye. Imagine taking a final exam in the future using Interent contact lenses to download all the answers under the nose of the professor! Internet contact lenses could change everything, from our way of life to the world economy itself.